We use cookies to ensure that we give you the best experience on our website. It will mean you have agreed with we would get cookies when you continue to see this website.
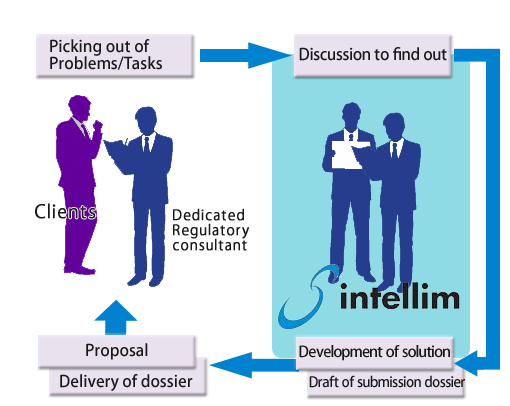
Our experienced regulatory experts support you regulatory affairs fro pharmaceuticals, medical devices and any other medical-related matters, survey and consulting from each phase of research to development and submission.
Our experienced expert support you various regulatory related matters. They understand exactly technical and market information through Database search, literature search, and hearing from external professionals.
intellim support you to conduct surveys to clinical practicians and end-users, literature search and others.
intellim’s unique Regulatory support services
And others. Please feel free to contact us.
For more information
If you have any comments, questions, please contact us.