We use cookies to ensure that we give you the best experience on our website. It will mean you have agreed with we would get cookies when you continue to see this website.
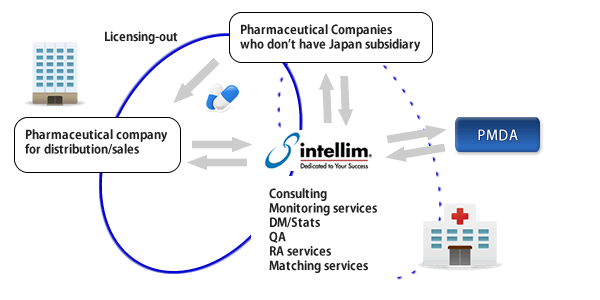
intellim takes a responsibility as a sponsor for your clinical trials even if you don’t have your office in Japan.
Our experienced personnel also supports you to find a company for licensing out.
※Definition of Article 15 in GCP
"In order to avoid any occurrence of harm to public health and hygiene with respect to the new investigational drug, and to enable the party to take any necessary actions to prevent such harm from spreading, all parties who do not have a presence in Japan, but wish to conduct clinical trials in this country, shall identify a party who has a presence in Japan, such as a representative of the Japan office of the foreign corporation, that has the capability of requesting clinical trials to be conducted on its behalf, and shall allow said party (hereafter to as the “In-Country Caretaker”) to carry out the necessary procedures relating to the clinical trial."
Before starting clinical trials
↓
During the trial
↓
After completion of the trial
For more information
If you have any comments, questions, please contact us.