In-Country Caretaker (ICC)
intellim works as "In-Country Caretaker" to do various required operations when you would like to import drug substances manufactured by foreign-based manufacturers. After the approval for import, intellim will be the contact window of foreign-based manufacturers, and support you from change control to notification of change or application of partial change. In addition, intellim will support to distribute Change Notification to all the pharmaceutical companies which use the relevant drug substance for their products.
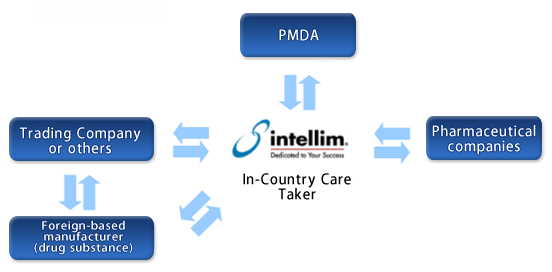
Our Services
- Preparation of documents for Master File (MF)
- Support to register MF as In-Country Caretaker (ICC)
- Archiving and management of data including supportive material/documents for MF.
- Creating communication plan with foreign-based manufacturer
- Amendment and renewal of registered MF
- Communication with Japan local pharmaceutical companies regarding MF (presentation of disclosed information)
- intellim also supports you to respond to PMDA related to MF when PMDA comes for inspection after a local pharmaceutical company submits the dossier for manufacturing using the relevant drug substance.
- Act as contact window for GMP Compliance Survey done by PMDA (Negotiation with foreign-based manufacturers)
- Change control after manufacturing approval, if necessary, intellim supports you from notification of change to application of partial change










