We use cookies to ensure that we give you the best experience on our website. It will mean you have agreed with we would get cookies when you continue to see this website.
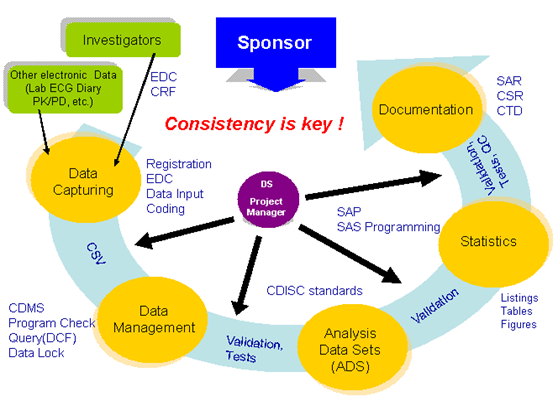
intellim established Data Sciences Department to unify Data Management Group and Statistical Analysis Group. Our new structure solves any issues which were occurred in their independent operative process or lack of coherent policy to deliverable.
intellim consolidates a sequence of operation including 1. Data Capturing, 2. Data Management, 3. Analysis Data Set, 4. Statistical Analysis Operation, 5. Documentation. It is a feasible solution to reach good cost performance.
In recent years, the progress of IT technology and globalization make environment surrounding clinical data change quickly. It is also required to flexible response to each country's regulation. Our Data Science Department always gets that information in advance of others, and adjust our structure for those changes.
| Scope of Business | Clinical Trial(Clinical Research) | PMS |
|---|---|---|
| Subject Registration(IWRS, FAX, Phone) | ○ | ○ |
| Randomization(including Sequential allocation by Minimization) | ○ | ○ |
|
EDC ・e-CRF development ・EDC system set-up ・CSV ・Training for sites and CRA ・Help desk |
○ | ○ |
| CRF development | ○ | ○ |
| Electronic Data(Lab, ECG, Diary, PK)Capturing | ○ | ○ |
| Data Entry/Correction | ○ | ○ |
| Scope of Business | Clinical Trial(Clinical Research) | PMS |
|---|---|---|
| Establishment of CDMS, its validation and maintenance | ○ | ○ |
| Data Management Plan(DMP) | ○ | ○ |
| Development and Establishment of Database | ○ | ○ |
| Computer System Validation(CSV) | ○ | ○ |
| Logical Check Program | ○ | ○ |
| CRF Check(Logical and Manual) | ○ | ○ |
| Coding(AE, clinical history, medication) | ○ | ○ |
| Re-survey(Query, DCF) | ○ | ○ |
| AE Matching | ○ | |
| Database Lock | ○ | ○ |
Features
・Standard Data Administration which is not depended by type of CDMS/EDC System.
・Use of IT Environment conformed with 21CRF Part11 and ER/ES Requirement
・Global EDCs (Viedoc™, Rave®) are proposable according to sponsor's requests.
・Proposal of Process and deliverable conformed with CDISC and others which are industry-wide standard.
・Highly value-added information to support Clinical Development Project
| Scope of Business | Clinical Trial(Clinical Research) | PMS |
|---|---|---|
| Specification of Analysis Data Set | ○ | ○ |
| Development of Analysis Data Set (Program and Verification) | ○ | ○ |
| Compliance with CDISC Standard | ○ | ○ |
| Preparation for material of case conference | ○ | ○ |
Features
・Conversion of Study Data captured by CRF/EDC to SDTM format
・Creation of ADaM based on SDTM
・Creation of Mapping Specification
・Creation of Unique Analysis Data Set for purpose of actual analysis
・Use of SAS for above mentioned operations
| Scope of Business | Clinical Trial(Clinical Research) | PMS |
|---|---|---|
| Consulting for Statistical Analysis | ○ | ○ |
| Planning of # of subjects | ○ | ○ |
| Development of Statistical Analysis Plan(Chart Layout) | ○ | ○ |
| Development of Statistical Analysis Specification | ○ | ○ |
| Development and Validation of Program for Analysis | ○ | ○ |
| Statistical Analysis(Output of Chart) and its Verification | ○ | ○ |
| Operation of IDMC(Interim Analysis) | ○ | |
| Key Open(Preparation of materials, Real time Analysis) | ○ | |
| Pharmacokinetic Analysis(PK/PD, Bio-equivalency) | ○ |
Features
・Our experienced trail statistician develops Statistical Analysis Plan and Statistical Analysis Report.
・SAS Global Certified Programmers program for you and verify it.
・Statistical Consulting for proposal of model for a new trial design.
・Supporting for various trial phase form clinical pharmacology study to verification study.
| Scope of Business | Clinical Trial(Clinical Research) | PMS |
|---|---|---|
| Development of Protocol (a part of statistical analysis) | ○ | |
| Creation of Statistical Analysis Report | ○ | ○ |
| Creation of Clinical Study Report(a part of statistical analysis) | ○ | |
| Preparation of ISS/ISE | ○ | |
| Preparation of CTD(a part of statistical analysis) | ○ | |
| Preparation of periodical safety report material, submission material for re-Examination | ○ |
Features
・Our staff members who have pharmacology background and statistics support you to generate various kinds of documents
・Our coherent operation structure will find mistakes, and it can make a corrective action promptly
・Preparation of form layout for final documents
・Ancillary Analysis (integrated analysis, meta analysis and others) is available with CTD, ISS/ISE and others
For more information
If you have any comments, questions, please contact us.