We use cookies to ensure that we give you the best experience on our website. It will mean you have agreed with we would get cookies when you continue to see this website.

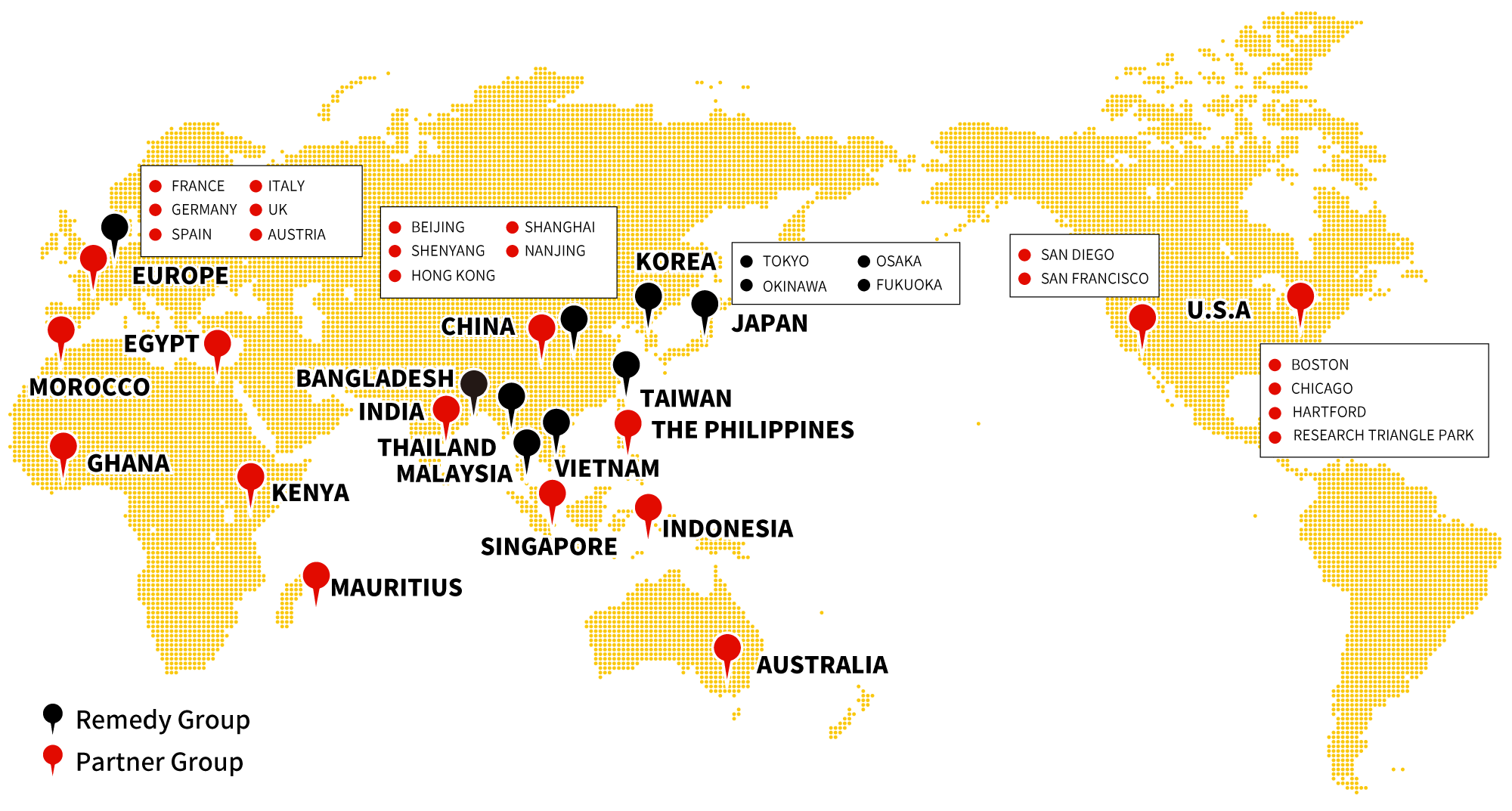
intellim provides full-scope support for regulatory affairs and various other drug development activities in Asian countries.
Regulatory Affairs & Medical Writing
intellim provides Regulatory related service for pharmaceuticals and medical devices, medical writing services, various survey services and consulting services for you.
Our experienced and educated CRAs conduct your clinical study with absolute accuracy, without a hindrance.
intellim takes a responsibility as a sponsor for your clinical trials even if you don't have your office in Japan.
Our Quality Assurance Team which is independent from Clinical Development Division is involved in GCP Audit. They provide audit services and consulting services for improvements after self-inspection.
intellim provides Pharmacovigillance services and service for compiling and analyzing the data from Treatment Out come Study after post marketing.
intellim consolidates a sequence of operation including Data Capturing, Data Management, Statistical Analysis and Documentation to provide high quality data for you.
| Japan |
China/ Hong Kong |
South Korea | Taiwan | Malaysia | Thailand | Vietnam | |
|---|---|---|---|---|---|---|---|
| Country Feasibility | ● | ● | ● | ● | ● | ● | ● |
| Clinical Development Consultation |
● | ● | ● | ● | ● | ● | ● |
| Pre IND meeting support | ● | ● | ● | ● | ● | ● | ● |
| IND dossier preparation | ● ICCC |
● | ● | ● | ● | ● | ● |
| IRB submission and negotiation with authorities |
● ICCC |
● | ● | ● | ● | ● | ● |
| Clinical Monitoring | ● | ● | ● | ● | ● | ● | ● |
| Safety reporting to the authority |
● Central |
● | ● | ● | ● | ● | ● |
| Data Management | ● JP/CH/KR/EN |
● JP/CH/KR/EN |
|||||
| Statistical Analysis | ● | ● | |||||
| Medical Writing | ● | ● | ● | ● | ● | ● | ● |
| NDA Support & Consulting | ● | ● | ● | ● | ● | ● | ● |
| PMS | ● | ● DIM |
● | ● |
For more information
If you have any comments, questions, please contact us.